Traits
Trait: Infection susceptibility (MAL)
Dr Haran Sivapalan
/
September 13, 2021

What are pathogens?
A pathogen is a microorganism that causes disease. This includes various:
- Bacteria – e.g. Streptococcus pneumoniae (which causes pneumococcal disease) or Mycobacterium tuberculosis (pictured in the title image, which causes TB).
- Viruses – e.g. influenza viruses, common cold viruses, SARS-CoV-2 (which causes COVID-19).
- Protozoa – e.g. Plasmodium (which is responsible for malaria).
- Fungi – e.g. Candida albicans (which causes thrush / candidiasis).
- Worms - e.g. hookworms (responsible for intestinal disease).
Pathogens can damage tissues in several different ways, such as by producing toxins or by directly invading and destroying cells.

Given these threats of tissue damage and disease, our body has a complex immune system that is designed to recognise, neutralise, and remember pathogens, thereby protecting us against infection.
You can read more about the various components of our immune system in the COVID-19: How do I boost my immune system? blog article.
KEY POINTS
- Pathogens are disease-causing microorganisms e.g. bacteria, viruses, fungi.
How does the body recognise pathogens?
Pathogens (e.g. bacteria, viruses) often produce molecules that are unique to them. For example, many bacteria have small tails or ‘flagella’ that are composed of a protein called flagellin. Flagellin therefore acts as a kind of molecular signature for certain bacteria.
Another example is lipopolysaccharide (LPS), a molecule found on the surface of Gram negative bacteria (e.g. E.Coli) that adds structural integrity to their outer membranes.
Flagellin, LPS and other molecules unique to certain pathogens (e.g. viral DNA sequences) are examples of PAMPs – pathogen-associated molecular patterns. PAMPs act as giveaways to our immune system that we have been invaded by harmful microorganisms.
So, how do we detect PAMPs on pathogens?
Cells of our immune system (e.g. white blood cells) have evolved specialised receptors – known as pattern recognition receptors (PRRs) – that can bind to PAMPs. Different types of PRRs bind to different types of PAMPs and go on to trigger different types of immune responses. In this way, we can tailor our immune responses to particular pathogens.

Source: Goulopoulou, S., McCarthy, C. G., & Webb, R. C. (2016). Toll-like receptors in the vascular system: sensing the dangers within. Pharmacological reviews, 68(1), 142-167.
One of the most important classes of PRRs are Toll-like receptors (TLRs). There are at least 10 different types of TLR in humans, each of which binds to a specific set of PAMPs.
For example, TLR5 binds to and is activated specifically by flagellin, whereas TLR4 is activated by LPS.
When a TLR is activated by a pathogen (or, more specifically, their pathogen-associated molecular patterns [PAMPs]), it triggers a signalling cascade which ultimately results in the switching on of genes that coordinate an immune response to neutralise the pathogen.
KEY POINTS
- Pathogens have specific molecular patterns (PAMPs) that identify them to the immune system as harmful organisms.
- Immune cells (e.g. white blood cells) have specialised receptors (including Toll-like receptors) that are capable of binding to the molecular patterns on pathogens.
- When PAMPs on pathogens bind to Toll-like receptors on immune cells, they trigger a signalling cascade that results in inflammation and the activation of the immune system.
What happens after pathogens are recognised?
As described in the previous section, specific molecules on pathogens (PAMPs) bind to and activate toll-like receptors (TLRs) found both on the surface of and inside immune cells.
Once PAMPs bind to TLRs, they trigger a chain of chemical reactions known as a signalling cascade. Signalling cascades are extremely important in biology and typically involve the serial activation of proteins, resulting in some sort of cell response.
In the case of certain TLRs (TLR1, 2, 4, 6), one of the first proteins to be activated in the signalling cascade is called TIRAP (Toll/interleukin-1 receptor domain-containing adaptor protein), which is also known as Mal.

Source: Goulopoulou, S., McCarthy, C. G., & Webb, R. C. (2016). Toll-like receptors in the vascular system: sensing the dangers within. Pharmacological reviews, 68(1), 142-167.
Once activated, TIRAP/Mal then goes on to activate molecules known as transcription factors. Transcription factors are molecules that switch genes ‘on’ or ‘off’. Two key transcription factors that are ultimately activated by TIRAP/Mal are: NF-κB and AP-1.
As illustrated in the diagram above, NF-κB and AP-1 switch on pro-inflammatory genes. This leads to the production of pro-inflammatory signalling molecules called cytokines, which cause inflammation and recruit immune cells (e.g. phagocytes) to neutralise and remove the pathogen.

As discussed in the Inflammation and IL-6 trait article, (acute) inflammation is a protective response to invasion by pathogens. It causes dilation and increased permeability of local blood vessels, allowing immune cells to quickly migrate towards pathogens; the release of cytokines help to mark the pathogen out for destruction by white blood cells; and the release of anti-microbial proteins help to destroy pathogens.
Although inflammation helps to protect against infection by pathogens, it’s worth noting that excessive inflammation can cause damage to our cells and tissues.
KEY POINTS
- Inflammation is a beneficial immune response that helps to neutralise and clear pathogens.
- When pathogens activate Toll-like receptors on immune cells, it triggers a signalling cascade that leads to pro-inflammatory genes being switched on.
- This causes the production of inflammatory signalling molecules (called cytokines) that promote inflammation, recruit immune cells and help co-ordinate an immune response to neutralise pathogens.
What is MAL?
The MAL (or TIRAP) gene codes for the Mal/TIRAP adaptor protein described in the previous section.
To recap briefly, Mal is one of the proteins in a signalling cascade that gets activated when pathogens bind to and trigger toll-like receptors (TLRs). Mal then goes on to activate other proteins, resulting in the switching on of inflammatory genes and stimulation of acute inflammation.
Studies suggest that variants of the MAL gene can affect levels of inflammation in response to pathogens. This, in turn, may affect susceptibility to infection. On this note, some MAL variants are linked to lower risk of infectious diseases such as malaria, tuberculosis, and pneumococcal disease.
- MAL gene variants
A SNP (Single Nucleotide Polymorphism) within the MAL (or TIRAP) gene, designated rs8177374, causes a C --> T change in the DNA code. This gives rise to two different MAL gene variants or ‘alleles’:
- The ‘C’ allele (also known as the ‘S’ allele).
- The ‘T’ allele (also known as the ‘L’ allele).
As we inherit genes in pairs (one from our mother, and one from our father), there are three possible genotypes:
- CC (also known as SS).
- CT (also known as SL).
- TT (also known as LL).
As we’ll explain in the following sections, the ‘T’ allele has been linked to a greater inflammatory response to infection.
Furthermore, the CT genotype has been linked with optimal levels of inflammation, which confers optimal resistance to infection.
KEY POINTS
- The MAL (or TIRAP) gene codes for a protein that is activated when Toll-like receptors on immune cells bind to PAMPs on pathogens.
- The MAL gene plays a key role in promoting inflammation in response to infection by pathogens.
- The rs8177374 SNP in the MAL gene creates two variants/alleles: 'C' and 'T'.
- Inheriting one copy of the 'T' allele (i.e. the CT genotype) is linked to moderate levels of inflammation and better resistance to infection.
How do MAL gene variants affect the inflammatory response to pathogens?
Studies suggest the ‘T’ allele (rs8177374) of the MAL or TIRAP gene is associated with a greater inflammatory response to pathogens.
In one study, human volunteers were infused with LPS (lipopolysaccharide). As described in the ‘How does the body recognise pathogens?’ section above, LPS, a molecule found on the surface membrane of several types of bacteria, is an example of a pathogen-associated molecular pattern (PAMP).
When LPS binds to and activates toll-like receptors (TLRs) on immune cells, it causes pro-inflammatory genes to be switched on, leading to the production of pro-inflammatory cytokines – signalling molecules that promote inflammation.
After infusing subjects with LPS, researchers then took blood samples at 30 minute intervals and measured levels of cytokines. In particular, they measured levels of TNF-α, IFN-ƴ, and IL-6, all of which are pro-inflammatory cytokines.

Source: Ferwerda, B., Alonso, S., Banahan, K., McCall, M. B., Giamarellos-Bourboulis, E. J., Ramakers, B. P., ... & Netea, M. G. (2009). Functional and genetic evidence that the Mal/TIRAP allele variant 180L has been selected by providing protection against septic shock. Proceedings of the National Academy of Sciences, 106(25), 10272-10277.
As shown in the diagram above, those carrying the ‘T’ (or ‘L) allele (i.e. those with the CT or SL genotype) had significantly higher cytokine levels after 60-90 minutes compared to non-carriers (i.e. those with the CC or SS genotype).
The researchers posited that a greater early inflammatory response in ‘T’ allele carriers is beneficial, as it affords better protection against infection by pathogens. Higher levels of pro-inflammatory cytokines would help pathogens to be marked, neutralised, and cleared more effectively.
As we’ll discover in the following section, carrying one copy of the ‘T’ allele (i.e. having the CT or SL genotype) has indeed been linked to greater resistance to infection.
Interestingly, however, the same cannot be said for people with two copies of the ‘T’ allele (i.e. those with the TT or LL genotype). In the aforementioned study, people with two copies of ‘T’ allele (TT genotype) had significantly higher cytokine levels than those with just one copy (CT genotype), suggestive of a greater degree of inflammation in response to pathogens.
Similarly, in a study of malaria patients, researchers found that those with the TT (or LL) genotype had significantly higher levels of the pro-inflammatory cytokine TNF-α, compared to those with the CT (or SL) genotype. This is illustrated in the graph below.

Source: Panda, A. K., Das, B. K., Panda, A., Tripathy, R., Pattnaik, S. S., Mahto, H., ... & Ravindran, B. (2016). Heterozygous mutants of TIRAP (S180L) polymorphism protect adult patients with Plasmodium falciparum infection against severe disease and mortality. Infection, Genetics and Evolution, 43, 146-150.
This greater inflammatory response in those with the TT genotype, however, is thought to be disadvantageous, as excessive inflammation can damage our own cells and tissues. For example, an extreme inflammatory response to infection can result in sepsis, a life-threatening condition whereby uncontrolled inflammation causes dysfunction of major organs. (We’ll elaborate on sepsis in a later section).
In line with this, the TT genotype has been linked to a increased risk of sepsis, and was associated with a 16 times higher risk of death (compared to the CC genotype) in malaria patients.
The effect of the ‘T’ allele of the MAL gene is a good example of what evolutionary biologists term a ‘heterozygote advantage.’ Whereas two copies of the ‘T’ allele (i.e. the homozygous TT genotype) causes excessive inflammation, which can harm health and survival, the ‘T’ allele has persisted in the gene pool because inheriting one copy (i.e. the heterozygous CT genotype) confers greater resistance to infection. By causing moderate levels of inflammation and moderate release of pro-inflammatory cytokines, heterozygotes (those with the CT genotype) are better able to detect, neutralise, and clear pathogens compared to homozygotes (those with either the TT or CC genotypes).
KEY POINTS
- Inflammation helps to neutralise and clear pathogens.
- Excessive inflammation, however, can damage the body's own tissue.
- The T allele of the MAL gene is linked to a greater inflammatory response to infection.
- Those with the CT and TT genotypes produce higher amounts of pro-inflammatory cytokines in response to pathogens.
- The heterozygous CT genotype has been linked to moderate levels of inflammation in response to infection, which may optimal for clearing pathogens and fighting infection.
- The TT genotype is linked to higher levels of inflammation than the CT genotype, which may be disadvantageous as excessive inflammation can damage tissues.
- The 'T' allele may have been selected for by evolution as it confers greater resistance to infection in the heterozygous genotype (CT) - this is known as the "heterozygote advantage."
How do MAL gene variants affect susceptibility to infection?
People with one copy of the ‘T’ (also known as ‘L’) allele of the MAL gene have been shown to be more resistant to infection by various pathogens.
Studies have shown those with the CT (or SL) genotype (rs8177374) are less susceptible to a variety of infectious diseases, including:
- Malaria
- Pulmonary TB
- Invasive pneumococcal disease
- H. pylori infection.
As described in the previous section, this protective effect of the heterozygous CT genotype is likely due a moderately increased inflammatory response to pathogens conferred by one copy of the ‘T’ allele. The release of an intermediate amount of pro-inflammatory cytokines, which occurs in response to pathogens binding to and activating toll-like receptors (TLRs), may enhance the clearance pathogens and protect against infection.
By contrast, people with two copies of the ‘T’ allele (i.e. the TT genotype) may experience an excessive inflammatory response to pathogens, which, due to damage inflicted on the body’s own tissues, may increase susceptibility to some infections.
Let’s take a deeper look at what some of the studies show.
- Malaria
Malaria is a tropical disease spread by mosquitoes. It is caused by infection by one of five species of Plasmodium – single-celled parasites that are spread to humans through the bites of female Anopheles mosquitoes.

Symptoms of malaria include fever, headache, muscle pains, diarrhoea, and vomiting. In more severe cases, the Plasmodium parasite can destroy red blood cells, leading to anaemia, or cause the formation of blood clots that block blood flow to the brain (a complication known as cerebral malaria). Malaria can also severely damage other major organs such as the kidneys and liver, leading to multi-organ dysfunction.
Various studies have linked the CT (or SL) genotype to a reduced risk of malaria. Furthermore, the CT genotype is associated with less severe malaria infection. On this note, a study of Indian malaria patients found that those with the CT genotype were significantly less likely than those with the CC genotype to have severe malaria or multi-organ dysfunction.
The researchers also looked at the malaria patients’ prognosis, comparing genotypes of people who died to those of who survived. Compared to those with the CC genotype, individuals with the heterozygous CT genotype were 69% less likely to die from malaria complications.
Interestingly, those with the TT genotype were 16.45 times more likely to die (than those with the CC genotype). This observation may be due to an excessive inflammatory response in those with the TT genotype, which increases the risk of life-threatening complications.
- Pulmonary TB
Tuberculosis (TB) is a bacterial infection caused by Mycobacterium tuberculosis. Pulmonary TB refers to when the bacteria infect the lungs, causing symptoms such as a persistent, productive cough and breathlessness.
One study analysed the genotypes of 700 subjects from Italian, Romanian and Ukrainian backgrounds who either had pulmonary TB or were healthy controls. The study found that, compared to other genotypes, the CT genotype of the MAL gene was associated with 54% lower odds of having pulmonary TB.
The same study also took samples of immune cells and incubated them with heat-killed Mycobacterium tuberculosis in order to model a real-life inflammatory response to infection.
Interestingly, and in line with the heterozygote advantage theory described earlier, immune cells from people with the CT genotype produced intermediate levels of pro-inflammatory cytokines compared to other genotypes. This tentatively suggests that having moderate levels inflammation in response to Mycobacterium tuberculosis infection could help protect against pulmonary TB.
- Invasive pneumococcal disease
Invasive pneumococcal disease (IPD) is the collective name for infections with the Streptococcus pneumoniae bacteria, whereby the bacteria enter the bloodstream or another normally sterile site (e.g. cerebrospinal fluid surrounding the brain).

IPD can lead to infection of several different tissues around the body, causing severe complications such as meningitis (infection of meninges surrounding the brain), peritonitis (infection of inner abdominal lining) and pericarditis (infection of sac surrounding the heart).
A case-control study of 6,106 subjects from British, Kenyan, Vietnamese and West African populations looked at whether having the heterozygous CT genotype was associated with a lower risk of various diseases, including IPD.
In the British cohort, it found that those with the CT genotype had a 35% lower risk of IPD compared to individuals with the CC genotype.
By contrast, those with the TT genotype were found to have a 2.39 times higher chance of having IPD. Again, this potentially suggests that the increased inflammatory response observed (from other studies) in TT individuals may enhance susceptibility to infection.
- H.pylori infection
Helicobacter pylori (H.pylori) is a bacterium that can infect and damage the lining of the stomach and small intestine.
Although often asymptomatic, H.pylori infection can cause inflammation of the stomach lining (gastritis) or open sores on the stomach and intestinal lining (peptic ulcer).

Studies have shown that the heterozygous CT genotype of the MAL gene may be protective against H.pylori infection. For example, an Italian case-control study, which compared MAL genotypes of individuals with confirmed H.pylori infection to those of healthy controls, found that the CT genotype was associated with a 44-47% lower risk of H.pylori infection.
KEY POINTS
- The heterozygous CT genotype has been linked to reduced susceptibility to infection.
- Studies have associated the CT genotype with a lower risk of malaria, TB, pneumococcal disease and H.Pylori infection (compared to other genotypes).
- The TT genotype has been linked to a higher risk of invasive pneumococcal disease (IPD) and a greater risk of death in malaria patients.
What is sepsis?
Sepsis is a life-threatening condition whereby the body has an extreme inflammatory response to infection. Bacterial infections are responsible for most sepsis cases, although other pathogens (e.g. viruses, fungi) can also cause sepsis.
Bacterial infections of the lung (e.g. pneumonia), skin (e.g. surgical wound infection), gastrointestinal tract, and urinary tract are the most common causes of sepsis.

The clinical signs of sepsis include:
- Fever, with or without shaking chills (temperature of > 38 °C or < 36 °C)
- Systolic blood pressure: <100 mm Hg.
- Heart rate > 90 bpm
- Respiratory rate ≥ 22 bpm
- Confusion / altered consciousness
- Warm or cold skin
Without urgent treatment, sepsis can lead to organ failure and death.
KEY POINTS
- Sepsis is the body's extreme immune response to infection.
- Sepsis is characterised by severe, widespread inflammation.
- Sepsis is a life-threatening condition that can lead to organ failure and death without timely treatment.
What happens during sepsis?
As explained in the “What happens after pathogens are recognised?” section, molecular signatures on pathogens (pathogen-associated molecular patterns or PAMPs) can bind to and stimulate various receptors on immune cells (e.g. Toll-like receptors), causing the activation of signalling pathways that lead to inflammation.
In sepsis, several of these signalling pathways become activated simultaneously, which switches on pro-inflammatory genes en masse, leading to overwhelming levels of inflammation.
As tissues and cells get damaged, they also release molecules called damage-associated molecular patterns (DAMPs), which further activate inflammatory pathways. This also contributes to the excessive inflammation or “hyperinflammation” seen in sepsis (see diagram below).
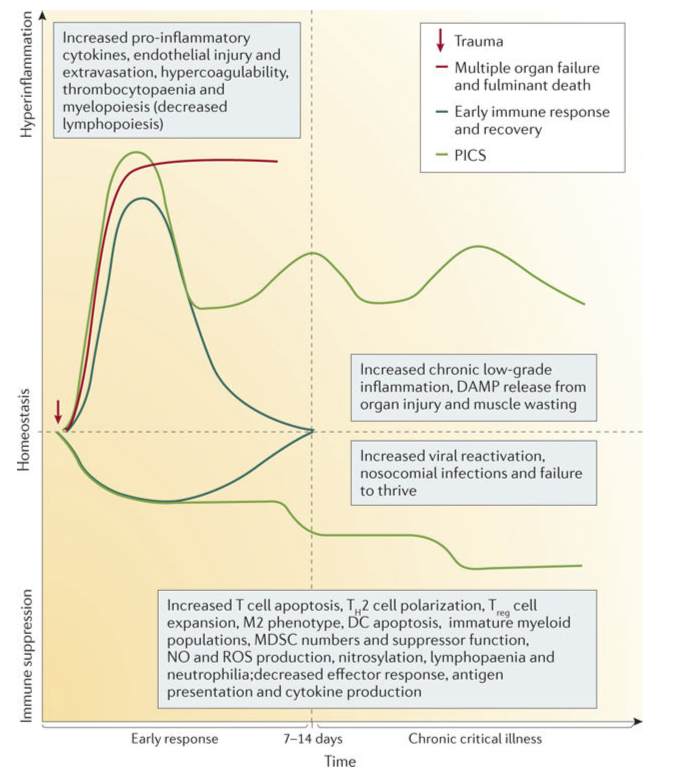
Source: Hotchkiss, R. S., Moldawer, L. L., Opal, S. M., Reinhart, K., Turnbull, I. R., & Vincent, J. L. (2016). Sepsis and septic shock. Nature reviews Disease primers, 2(1), 1-21.
Although moderate inflammation can help to neutralise and clear harmful pathogens, excessive inflammation can also damage the body’s own tissues. In sepsis, tissue damage from severe inflammation can compromise the function of key organs, such as the kidneys or liver. The term multi-organ dysfunction is used to describe the situation when sepsis causes two or more key organs to be unable to function effectively without medical intervention.
In a moderate inflammatory response, dilation (vasodilatation) and increased leakiness/permeability of blood vessels allows immune cells in the bloodstream to more easily access and clear pathogens from sites of infection. In sepsis, however, inflammation causes widespread, excessive vasodilatation, which leads to a drop in blood pressure. (You can read more about vasodilatation and blood pressure here).
Due to this drop in blood pressure, blood may fail to adequately perfuse key organs. When this happens, it is referred to as septic shock.
Excessive inflammation in sepsis can also activate platelets and clotting factors in the blood, making it more likely to form blood clots (hypercoagulability) that compromise blood flow to organs. Perhaps counterintuitively, this can lead to excessive bleeding, as the bloodstream ‘runs out’ of clotting factors and platelets (known as thrombocytopaenia).
After an early inflammatory response to infection that clears pathogens, levels of inflammation normally subside, allowing tissues to heal. In sepsis, however, inflammation continues and can lead to organ dysfunction and, eventually, death. This is shown in the red line of the above diagram.
For patients who survive sepsis, many may experience continued chronic inflammation and/or suppression of the immune system. This is known as PICS (persistent inflammation, immunosuppression and catabolism syndrome) – which is illustrated by the green line in the above diagram.
KEY POINTS
- Sepsis involves overactivation of inflammatory pathways in response to infection by pathogens.
- Excessive inflammation (hyperinflammation) causes tissue damage, which can eventually prevent organs from functioning effectively.
- Sepsis may cause blood pressure to drop significantly, which means organs cannot be adequately perfused with blood. This is known as septic shock.
- Some sepsis patients may experience continued high inflammation levels and/or immunosuppression long after initial infection.
How do MAL gene variants affect risk of sepsis?
The TT genotype (rs8177374) of the MAL gene has been tentatively linked to a higher risk of sepsis.
For example, a study of German subjects compared the frequency of MAL genotypes in sepsis patients against healthy controls. The TT genotype was found to be overrepresented in sepsis patients, although at borderline statistical significance.
Another study analysed the MAL genotypes of 166 patients on ventilators for bacterial sepsis and pneumonia. The researchers found that those with the TT genotype had more severe illness, as indicated by a higher clinical pulmonary infection score and a trend towards lower oxygenation of tissues.
Although larger studies are required to fully elucidate the relationship between sepsis and MAL genotype, there are plausible reasons for how the TT genotype could increase the risk of sepsis. As explained previously, the TT genotype is linked to a greater inflammatory response and higher levels of pro-inflammatory cytokines following infection.
It is possible that TT individuals with this greater inflammatory response are at a higher risk of progressing into the exaggerated, widespread and dysregulated inflammatory response that characterises sepsis.
KEY POINTS
- The TT genotype of the MAL gene has been linked in some studies to a higher risk of sepsis.
- Increased inflammation in response to infection may enhance the risk the risk of sepsis in TT individuals.
Your Infection susceptibility (MAL) trait
Your Infection susceptibility (MAL) trait looks at variants of the MAL (also known as TIRAP) gene created by the rs8177374 SNP. Depending on your DNA results, you will be classified into one of three groups:
- Average protection against infection - you have the CC genotype linked to average levels of inflammation in response to infection.
- Increased resistance to infection – you have the heterozygous CT genotype linked to moderate levels of inflammation in response to infection and greater resistance to various infectious diseases (e.g. malaria, TB).
- Increased inflammation risk – you have the TT genotype linked to higher levels of inflammation in response to infection. Excessive levels of inflammation may increase the risk of sepsis and severe outcomes after infection.
To find our your result, please login to truefeed.




.png)
